EASEE® (Epicranial Application of Stimulation Electrodes for Epilepsy) is a new device to reduce the number of seizures for people with drug-resistant focal seizures. It is available on the NHS but currently, not all hospitals offer the procedure.
How does it work?
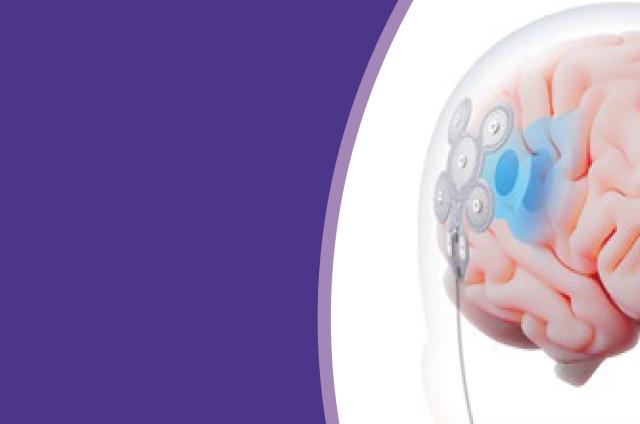
EASEE® uses different types of electrical pulses to reduce seizures and stabilise brain activity – almost like a ‘brain pace-maker’. These pulses help to stop the seizures or make them less severe.
The device can be adjusted to send the right amount of electrical pulses for each person. So, this makes the treatment very personalised.
The device is thin and about 5cm long. It is placed under the scalp, over the epileptic focus (where the seizure starts), through a cut in the skin.
A battery is placed under the skin on the chest. This is connected to the electrode with a thin wire running under the skin.
How can EASEE®help?
EASEE® is used alongside your anti-seizure medication (ASM) to help reduce seizures over a period of time. In 2023, two trials involving 33 patients, saw a reduction in their seizures after using EASEE®. In six months, 53% of people saw a reduction of 50% in seizure frequency. After two years, 65% showed a 50% reduction.
People in the two year study did not experience any serious complications or side effects with the device. And 81% of people who started treatment carried on with it for the two years of the study.
What are the benefits of EASEE®?
- The device is placed under the scalp without major surgery.
- The electrical pulses can be adjusted for each person’s needs.
- By reducing seizures, EASEE® can help people live more normal lives and can improve their quality of life.
- The device is thin and discreet. You should not feel it while going about your daily activities.
- If the device needs to be removed, it can be done so completely and safely.
- You can have Magnetic Resonance Imaging (MRI) scans even after the device is implanted. However, you should notify the MRI department that you have the device as there will be safety precautions.
What are the risks of EASEE®?
- Initially there may be be some bleeding, swelling, or pain in the area but this should go away on its own.
- There may be some risk of infection which may need antibiotics or for the device to be removed.
- In the unlikely event that the device or wires move there may be a need for another procedure.
- The battery is designed to last between three and five years but another procedure will be required to replace it.
- The device does not work for everyone.
- Because the device is inserted under general anaesthetic (GA), there is the risk of complications or side effects from the GA.
Who can have EASEE®?
This treatment may be available for you if:
- your seizures come from one focused area of the brain;
- you have tried at least two medications that haven’t worked for you;
- there is more than one focus for the seizures but it is possible to identiy the dominant one; and
- you are not suitable for eplilepsy surgery.
How do I get the device?
EASEE® is available on the NHS but not everywhere. You will need to speak to your neurologist to discuss whether the treatment is suitable for you and where you may be able to go to get it.
What happens after the procedure?
Following implantation you will go home and wait for a month before the device is switched on. This is to allow for complete healing. It is simple and easy for your specialist to switch the device on.
You will need a review with your specialist doctor at three months, although it may be earlier or later than this depending on your doctor’s decision. Everyone is different, so it may take a few months to notice any effects.
There is then a six month review. Sometimes, the electrical settings on the device might need to be adjusted for better results at this review.
Further information
Precisis
easee.precisis.de/en/patients-and-caregivers/
Precisis are the manufacturers of the EASEE® device and they have further information.
University College London Hospitals (UCLH)
uclh.nhs.uk/patients-and-visitors/patient-information-pages/neurosurgery-guide-epilepsy-patients-considering-implantation-easee-device
UCLH offer the procedure and have further information.
Epilepsy Society is grateful to Ben Driver, Senior Clinical Sales Manager: UK & Ireland, Precisis, and Dr F J Rugg-Gunn, Consultant Neurologist & Honorary Associate Professor, Clinical Lead, Chalfont Centre for Epilepsy, who reviewed this information.
Information produced: July 2025. Review date: July 2027
Download this information
For a printed copy contact our Helpline.
Brain scans
In order for a person to be suitable for surgery, it is necessary to confirm that seizures are arising from one part of the brain and that it is safe to remove this part. This requires many tests including MRI brain scans.
Epilepsy surgery
Brain surgery or neurosurgery is one way of treating epilepsy. Certain criteria have to be met and tests have to be done to assess suitability.
Anti-seizure medication (ASM)
This information is for both adults and children with epilepsy and answers some questions you might have about anti-seizure medication. Where we talk about controlling seizures or ‘seizure control’ we mean stopping seizures from happening.