Charities call for pause to new prescribing practices around valproate
The UK epilepsy organisations listed below are joining together in an urgent call for a decision by the Medicines and Healthcare products Regulatory Agency (MHRA) and Ministers to be paused immediately.
Martin and the King share their sense of family loss
When Martin Evans sent a letter of condolence to King Charles III, following the death of the Queen, he did not expect to receive a signed letter in return.
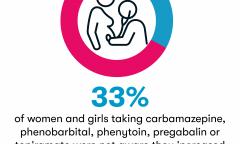
Third of women unaware of meds risk to unborn babies
A third of women taking some of the most commonly prescribed anti-seizure medications are unaware of the risks they pose to unborn babies, according to a new survey. And this figure rises to over half among women under 24.
International Women's Day 2023
Today is International Women's Day, a global celebration of women's rights and gender equality.
Voting: what to do if you don't have photo ID
The local elections in May will be the first time that all voters in England need to have a form of photo ID in order to cast their ballot in person. For those without photo ID, you can apply for a free Voter Authority Certificate.
Cenobamate for treating focal onset seizures in epilepsy
Cenobamate (Ontozry) is available on the NHS as a possible treatment for focal onset seizures with or without secondary generalised seizures in adults with drug-resistant epilepsy that has not been adequately controlled with at least 2 medicines.